Abstract
Introduction: Ibrutinib is extensively metabolized by cytochrome P450 CYP3A and drug interactions pose a considerable challenge with its use in B-cell malignancies. Patients with chronic lymphocytic leukemia (CLL) are often older with multiple comorbidities and complex medication histories. In this study, we aimed to evaluate the impact of drug interactions, in particular CYP3A inducers and inhibitors, on survival in patients with CLL treated with ibrutinib in routine clinical care.
Methods: We conducted a population-based cohort study using linked administrative healthcare databases from Ontario, Canada. All Ontario residents aged ≥66 years with CLL started on ibrutinib between January 2007 and March 2019 were identified and followed from the date of first ibrutinib prescription (index date) until March 1, 2020. The observation window was terminated in the event of death, no ibrutinib prescription for 120 days, or at the time of allogenic stem cell transplant, whichever occurred first. Cumulative medication exposure (30-day increments) to concomitant CYP3A inducers and/or CYP3A inhibitors were examined from 90 days prior to index date to the end of the observation window as a time-varying covariate. A comprehensive list of CYP3A inducers and inhibitors were obtained from the FDA (2020) and Flockhart Table of Cytochrome P450 Drug Interactions (Flockhart et al., 2021). The primary outcome was overall survival (OS). Cox regression, controlling for comorbid disease burden (aggregated diagnosis groups; ADG), ibrutinib dose (full vs. reduced) and sociodemographic factors (age, sex) examined the relationship between medication exposure and OS.
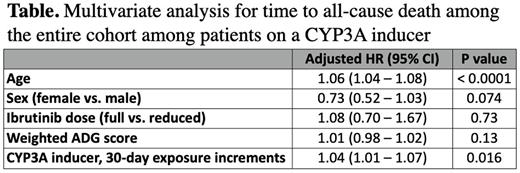
Results: We identified 642 patients with CLL treated with ibrutinib in Ontario between January 1, 2007 and March 1, 2019. The median age was 74 years (interquartile range (IQR), 69 - 80), 34.4% were female with a median weighted ADG score of 17 (IQR, 8 - 25), indicating a high burden of comorbidities in our cohort. At baseline, 563 (87.7%) patients received full-dose ibrutinib and 372 patients (57.9%) were on ibrutinib as first-line therapy. The median follow-up time from ibrutinib initiation was 2 years (IQR, 1.3 - 3.0) with 162 deaths (25.2%) and the median OS was not reached. During the study period, 131 patients (20.4%) received a concomitant CYP3A inducer, and 179 patients (27.9%) received a concomitant CYP3A inhibitor. On univariate analysis, we identified an association of increased risk of all-cause death with increased age (hazard ratio [HR] 1.06, 95% confidence interval [CI] 1.04 - 1.08, p <0.0001), exposure to a concomitant CYP3A inducer (HR 1.04, 95% CI 1.01 - 1.07, p = 0.009), and greater comorbid disease burden (HR 1.02, 95% CI 1.00 - 1.03, p = 0.01). Exposure to concomitant CYP3A inhibitors was not significantly associated with all-cause death (HR 1.00, 95% CI 0.98 - 1.02, p = 0.84). Multivariable analysis adjusting for age, sex, comorbid disease burden and ibrutinib dose (full vs. reduced) demonstrated an increased risk of death for patients on a concomitant CYP3A inducer (HR 1.04 for each 30-day increase in exposure, 95% CI 1.01 - 1.07, P = 0.016; see table) whereas CYP3A inhibitors had no significant effect on all-cause death (HR 0.99, 95% CI 0.97 - 1.01, P = 0.43). The most common cause of death for patients on a concomitant CYP3A inducer, where known, was CLL (54.9%).
Conclusions: Despite known drug interactions, CLL patients on ibrutinib and concomitant CYP3A inducers/inhibitors is common in routine clinical care. We demonstrate an association between CYP3A inducer exposure and an increased risk of death, which may be in part due to decreased ibrutinib levels. Our study highlights the importance of pharmacovigilance. Further analyses are underway to better understand the impact of drug interactions on ibrutinib treatment complications
Disclosures
Prica:Kite-Gilead: Honoraria; Astra-Zeneca: Honoraria. Thawer:Roche: Current Employment. Mozessohn:Abbvie: Current holder of stock options in a privately-held company; Bristol Myers Squibb: Current holder of stock options in a privately-held company; Johnson & Johnson: Current holder of stock options in a privately-held company; Merck and Co: Current holder of stock options in a privately-held company; Novo Nordisk: Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal